12/02/2019
The function of patent drawings is to aid understanding of an invention, but are they fit for purpose? Embodiments of a mechanical invention may be easier to explain and understand as a drawing compared to a lengthy description. The European Patent Office (EPO) considers good quality drawings as very important for the correct disclosure of an invention (Guide for applicants, Drawings). Scientific data necessary to support biotech and pharmaceutical inventions are also provided as “drawings”. However, these data do not serve the same purpose as mechanical drawings and do not naturally conform to the formal drawing requirements for technical illustrations of mechanical objects. However, this difference is not reflected in the formal drawing requirements of the EPC or PCT. We ask therefore, whether the formal drawings requirements deserve a re-think.
 The formal requirements for the form in which patent drawings of patent applications are to be presented are provided by Rule 11 PCT and Rule 46 EPC. At a functional level, the drawings, like all other parts of the application, must be “presented as to admit of direct reproduction by photography, electrostatic processes, photo offset, and microfilming, in any number of copies” (Rule 11(a) PCT). The special requirements relating to drawings are provided by Rule 11.3 (a)-(n)PCT and Rule 46 EPC.
The formal requirements for the form in which patent drawings of patent applications are to be presented are provided by Rule 11 PCT and Rule 46 EPC. At a functional level, the drawings, like all other parts of the application, must be “presented as to admit of direct reproduction by photography, electrostatic processes, photo offset, and microfilming, in any number of copies” (Rule 11(a) PCT). The special requirements relating to drawings are provided by Rule 11.3 (a)-(n)PCT and Rule 46 EPC.
Are data “drawings”?
From a quick perusal of the special requirements relating to drawings, it rapidly becomes apparent that these provisions are drafted with mechanical inventions in mind. In fact, the very phrase “drawings” seems inappropriate for the types of graphical illustration provided in support of other types of invention, particularly those in the software, biotechnology and pharmaceutical fields. The EPC (but not PCT) addresses this problem somewhat by stipulating that “flow sheets and diagrams shall be deemed to be drawings” (Rule 46 (3) EPC).
However, the problem goes beyond the inappropriateness of the term “drawing” for scientific data. Take for example the first provision of Rule 11 PCT and Rule 46 EPC “(a) Drawings shall be executed in durable, black, sufficiently dense and dark, uniformly thick and well-defined, lines and strokes without colorings”. This seems to be a sensible requirement for drawings taking the form of technical illustrations of a mechanical object. However, the types of “drawings” required to support the claims of a biotechnology or pharmaceutical generally take the form of data. If you have, for example, a claim in the second medical use form: “Drug X for the treatment of disease Y”, the applicant is required to provide sufficient data demonstrating the plausibility of the treatment effect. This may be presented, for example, as a bar chart showing a treatment effect in various patient groups over time.
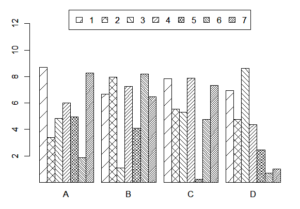 Scientific data are not suited for representation with black lines only. Different bars on a bar chart may be distinguished from each other by colour or colour intensity (e.g. different shades of grey). However, colours and shading are not permitted under Rule 11 (a) PCT. In order to satisfy the formal drawing requirements, it seems therefore that the shading must therefore be replaced by ever increasingly complicated types of cross-hatching. It in fact appears from Rule 11 PCT that graphs in general are not looked on favourably, given that Rule 11 (f) PCT states that a scale (even a graphical one) should only be drawn in “exceptional” cases.
Scientific data are not suited for representation with black lines only. Different bars on a bar chart may be distinguished from each other by colour or colour intensity (e.g. different shades of grey). However, colours and shading are not permitted under Rule 11 (a) PCT. In order to satisfy the formal drawing requirements, it seems therefore that the shading must therefore be replaced by ever increasingly complicated types of cross-hatching. It in fact appears from Rule 11 PCT that graphs in general are not looked on favourably, given that Rule 11 (f) PCT states that a scale (even a graphical one) should only be drawn in “exceptional” cases.
The EPO by contrast, has attempted to address the issue of the unsuitability of representing scientific data with black lines only. Whilst Rule 46 EPC stipulates that only black lines may be used, the EPO Guidelines for Examination indicate that “the use of shading in figures is allowed provided this assists in their understanding and is not so extensive to impede legibility” (A-IX 7.2).
What about photographs?
Scientific data may also take the form of photographs. Western blots and immuno-fluorescence are standard techniques for detecting proteins, and both type of data are often presented as photographs. In the field of 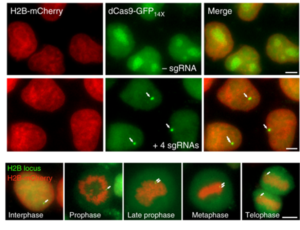 genetic modification, it is also usual to provide photos of the resulting modified organism so as to show the effect of a genetic modification on a physical trait. Does it make sense to execute these data “in durable, black, sufficiently dense and dark, uniformly thick and well-defined, lines and strokes without colorings”?
genetic modification, it is also usual to provide photos of the resulting modified organism so as to show the effect of a genetic modification on a physical trait. Does it make sense to execute these data “in durable, black, sufficiently dense and dark, uniformly thick and well-defined, lines and strokes without colorings”?
The PCT applicant’s guide indicates that photographs may be submitted: “[t]he PCT makes no provision for photographs. Nevertheless, they are allowed where it is impossible to present in a drawing what is to be shown (for instance, crystalline structures). Where, exceptionally, photographs are submitted, they must be black and white…Color photographs are not accepted, nor are color drawings” (PCT applicant’s guide, 5.159).
 The EPO Guidelines for Examination similarly indicates that photographs may be permitted “where it is impossible to present in a drawing what is to be shown…Colour photographs are accepted, but will be scanned, printed and made available via file inspection only in black and white” (A IX 1.2).
The EPO Guidelines for Examination similarly indicates that photographs may be permitted “where it is impossible to present in a drawing what is to be shown…Colour photographs are accepted, but will be scanned, printed and made available via file inspection only in black and white” (A IX 1.2).
The current PCT and EPO guidelines thus seem out of step with a normal interpretation of the PCT and EPC formal drawing requirements (e.g. that drawings must be executed in durable black lines).
Where does this all leave the applicant?
If the EPO find that the drawings provided in an application do not satisfy the requirements of Rule 46 EPC, they issue an invitation to the applicant to remedy the deficiencies within 2 months of notification (Rule 48 EPC). If the defect noted by the EPO is not  remedied on time or properly, the application will be refused (Article 90 (5) EPC). To avoid refusal of the application, the applicant is therefore simply required to remedy any deficiencies found in the drawings. So what is the problem?
remedied on time or properly, the application will be refused (Article 90 (5) EPC). To avoid refusal of the application, the applicant is therefore simply required to remedy any deficiencies found in the drawings. So what is the problem?
Whilst the EPO guidelines do indicate that graphs and photos may be allowed, the lack of a specific provision in the EPC means that the extent to which scientific data will be expected to conform to the Rule 46 EPC requirements can be open to the whim of the EPO Receiving Section. Replacing data with drawings satisfying the requirements of Rule 46 EPC can also represents a significant burden (in terms of time and expense) for the applicant. Furthermore, the resulting black and white, cross-hatched and text-free figures are often worse at communicating the data than the original figures.
All of this raises the question of how necessary the requirements of Rule 46 EPC really are? If the purpose of data is to demonstrate the plausibility of a claimed effect, surely what matters is whether a skilled person would be able to read and understand the data? Given that data generally cannot be considered “drawings”, and often do not serve the same purpose as technical drawings of mechanical inventions, perhaps data should be submitted under a different category, akin to the supplementary data of a scientific article?
The issue of the lack of applicability of the formal drawing requirements to scientific figures may not be the most pressing issue with the International and European patent system. Perhaps this Kat has just spent one too many times trying to squeeze the square peg of scientific data into the round hole of the formal drawing requirements for patent applications. Nonetheless, with the number of biotech and pharmaceutical applications rising every year (up 1.5 and 8.1% respectively in 2017), and the increasing importance given to the data required to support these applications, perhaps both applicants and the public would benefit from a rethink of the requirements. Do other EPO patent practitioners have any thoughts?
A version of this article was first published on The IPKat.
This article is for general information only. Its content is not a statement of the law on any subject and does not constitute advice. Please contact Reddie & Grose LLP for advice before taking any action in reliance on it.

