28/04/2021
A few weeks ago, the Enlarged Board of Appeal of the European Patent Office (EPO) issued a decision on the extent to which computer-implemented simulations are patentable in Europe (previously reported here).
The confirmation that computer-implemented simulations should be considered in the same way as any other computer-implemented process could be significant for the industry that filed more European patents than any other in 2020: Medical Technology.
Use of Simulations in Medical Technology
Simulations have become integral to modern science and technology, particularly for Medical Technology.
They are useful for modelling complex physical systems that cannot be adequately described mathematically, and recent advances in computing and processing power have ensured that simulations are more reliable than ever before.
The speed and accuracy of modern simulations enables researchers to, for example, identify an optimum drug for a desired outcome at a fraction of the cost and time. Many simulations provide a design environment where medical devices can be tested and optimised before being physically implemented. By dispensing with physical hardware for the majority of the design process, simulations can reduce the cost and time of testing and developing a new product.
Another significant use of simulations is for teaching and learning. In the Medical Technology industry this typically enables practitioners to encounter real-world scenarios without a threat to life that enables them to test and improve their clinical skills in a controlled environment.
Patenting Simulations in Europe
Perhaps surprisingly considering simulations are fundamental to modern innovation, in Europe simulations are not guaranteed to be eligible for patent protection in the first place. This is because a European patent can only be granted for an “invention” that provides a technical solution to a technical problem. This definition of an “invention” does not cover programs for computers, mathematical methods and mental acts (which cover simulations in their purest form), as these are not considered to be inherently technical.
However, simulations that are implemented on a physical system such as a computer may be patented so long as they clear the usual hurdles of being new, involving an inventive step, and being industrial applicable.
G1/19
Determining the extent to which computer-implemented simulations could involve an inventive step by providing a technical effect was the principal task of the Enlarged Board in G1/19. In summary, the main conclusions of the Enlarged Board were:
- The assessment of inventive step for computer-implemented simulations, including simulations that are part of a design process, should be no different to any other computer-implemented invention
- To provide a technical effect, the simulation does not need to have a direct link to physical reality, and there is no need for the simulated system to be inherently technical
Before the G1/19 decision there was doubt as to whether a simulation could fulfil the EPO’s criteria of involving an inventive step without including a direct link to a physical process or system. This is because under European practice, a computer-implemented invention must produce a technical effect when run on a computer. Generally, a technical effect could be found in anything that goes beyond the “normal” physical interactions between computer software and computer hardware. This lead to the suggestion that a simulation could only be patentable if it provided a technical effect on a physical entity in the real world: for example, when the simulation controls a technical process, such a medical training simulation controlling clinical equipment.
However, the Enlarged Board indicated that there is no requirement for the simulation to have a direct link with external physical reality.
This is a significant finding for the Medical Technology industry, where many uses of simulations do not include a direct link to a physical device. For example, a bioinformatics simulation that analyses simulated biological processes may not have any interaction with external hardware. For simulations such as these to be patentable, G1/19 indicated that the simulation must be a reason for the function of the computer to be adapted, or if the outputs of the simulation have an impact on physical reality.
These criteria may still be difficult to fulfil, but the G1/19 decision should give applicants more confidence to file applications for medical simulations.
Patent Filings in Europe for bioinformatics simulations
It will be interesting to see whether a wave of new European patent applications will be filed for medical simulations in the wake of the G1/19 decision.
The three graphs below show the trend of patent applications published in the past few years in China, the US and Europe under the IPC category G16B5 (which relates to modelling or simulating biological systems and processes, particularly in bioinformatics). If the patent filing trends for bioinformatics simulations are an accurate barometer for patent filings for medical simulations generally, then it appears that the increased use of simulations in the Medical Technology industry is fuelling a corresponding dramatic increase in patent filings for those simulations.
In the graphs below, the red trend lines indicate annual figures with the grey trend lines indicating cumulative figures.
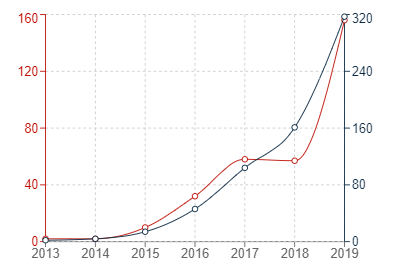
Graph 1: Chinese patent publications in G16B5
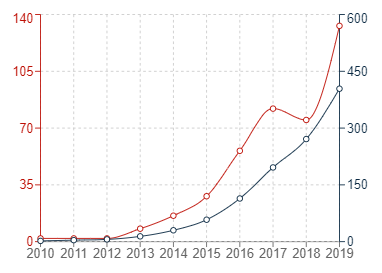
Graph 2: United States patent publications in G16B5
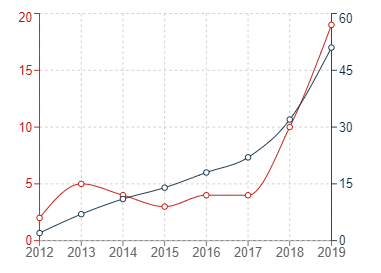
Graph 3: European patent publications in G16B5
It can generally be seen that the number of patent publications for inventions relating to bioinformatics simulations has increased significantly in all three jurisdictions within the past 10 or so years. The uncertainty surrounding patenting computer-implemented simulations may have contributed to the sluggish start in Europe, but it is very encouraging to see that Europe has also experienced a recent and dramatic uptick in new filings for medical simulations.
In addition, the number of Chinese-originating Medical Technology applications filed in Europe increased by an incredible 34% in 2020. Although the vast majority of European applications filed for Medical Technology continue to derive from Europe or the US (77%), Chinese applicants may play a pivotal role in driving future growth in the Medical Technology industry – including the development of medical simulations. It will be very interesting to see how these patent filing trends develop in the coming years.
At Reddie & Grose, our experienced multidisciplinary Medical Devices and Digital Healthcare team are on hand to advise on the IP challenges of the Medical Technology industry and to provide advice for seeking and securing patent protection.
This article is for general information only. Its content is not a statement of the law on any subject and does not constitute advice. Please contact Reddie & Grose LLP for advice before taking any action in reliance on it



