03/08/2021
In previous articles, we have considered how, in addition to patents, pharmaceutical and biopharmaceutical companies may achieve drug exclusivity in Europe through SPCs and regulatory data protection.
In this article, we consider how patents, SPCs, regulatory data protection and orphan drug market exclusivity may combine and interact to exclude competitors for as long as possible and maximise returns on substantial R&D investment.
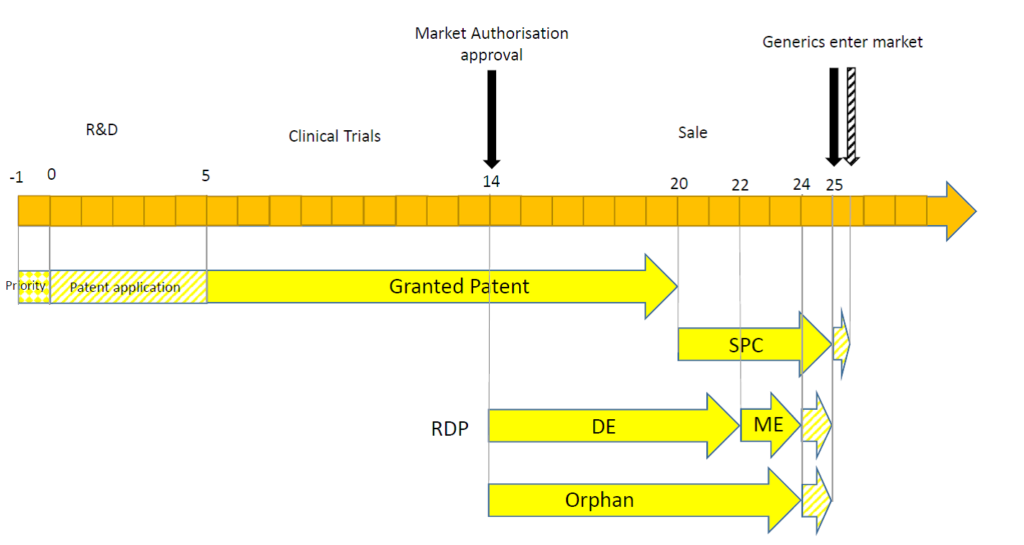
| -1 years |
| Shortly after beginning a research project, a compound (or class of compounds) may be discovered which is promising for treating a particular disease. At this stage, an initial priority patent application (such as a UK patent application) may be filed, directed to the compounds and their use. |
| 0 years |
| At the end of the priority year, a patent application is filed in Europe, claiming priority back to the earlier UK patent application. The date of filing of the patent application begins the maximum 20 year patent period. |
| 5 years |
| After five years of prosecution at the European Patent Office, a patent is granted and is validated in a number of European countries (in practice obtaining a granted patent may not take as long as this, or it may take longer than this depending on the patentability issues raised during prosecution). When the patent is granted, however, the compound is not yet approved by the appropriate regulatory authority (such as the European Medical Agency (EMA) or the UK’s Medicines and Healthcare products Regulatory Agency (MHRA)) and must first undergo clinical trials to establish safety and efficacy. So, although the patent provides an enforceable right, the monopoly cannot yet be exploited to generate revenue. |
| 14 years |
| The compound is found to be safe and efficacious and so is granted a Marketing Authorisation (MA). At this stage, the patent only has six years left of its 20 year term (calculated from the date at which the patent was filed). The date of grant of the MA begins a 10 year period of Regulatory Data Protection (RDP). The first eight years provides data exclusivity (DE) which means that generic companies cannot refer to the data from the original MA in order to support its own MA. After this period, generic companies, can rely on the data from the original MA in an abbreviated application for a MA. If the compound treats an orphan disease (diseases which are rare and would not be profitable without government assistance), the MA holder can obtain 10 years of exclusivity which may prevent competitors from filing their dossier for obtaining an MA, prior to its expiry. Orphan drug market exclusivity is independent of RDP, and so a compound designated for an orphan indication may benefit from market exclusivity which runs in parallel with RDP if the drug is also authorised (through a separate MA) for other indications. Within six months of the date of grant of the MA or six months from grant of the patent (whichever is later), an application for a Supplementary Protection Certificate (SPC) must be made. The SPC (once granted) confers substantially the same rights as the patent, but the scope of protection extends only to the compound authorised to be placed on the market, rather than extending the protection of the whole claim scope of the patent. The term of an SPC is equal to the period of time between the filing date of the patent and the date of the 1st MA in Europe, minus five years, but the maximum duration is five years. In this case, because the MA was granted 14 years after filing the patent, the SPC is eligible for the full five years of protection. |
| 20 years |
| The patent has expired. However, exclusivity is maintained by DE of the RDP and/or orphan drug market exclusivity, and by the SPC. |
| 22 years |
| The eight year DE term of RDP has expired. A generic company can now use the clinical trial data of the original MA in their applications, but they are not able to market their product because RDP provides for an additional two years market exclusivity (ME) afforded to the originator compound. |
| 24 years |
| The additional two year ME of RDP has expired. However, in some circumstances, the two year period can be extended by one year, affording a total of 11 years of RDP. This additional one year extension may be possible if, during the first eight years, the MA holder obtains an authorisation for one or more new therapeutic indications which are held to bring a significant clinical benefit in comparison with existing therapies. The 10 year period of orphan drug market exclusivity has expired. However, an additional two years of market exclusivity may be available for orphan drugs, should certain paediatric studies – a Paediatric Investigation Plan (PIP) – be undertaken. In the situation illustrated above, even if RDP and orphan drug market exclusivity has expired, generic companies are still not able to launch because the SPC is still in force. |
| 25 years |
| All forms of exclusivity have expired unless the SPC holder has managed to obtain a six month extension to the SPC term via a paediatric extension. A paediatric extension must be applied for at least two years prior to expiry of the SPC and can only be obtained if the SPC holder has a completed PIP. |
| 25.5 years |
| All possible forms of exclusivity have expired. |
The timeline above is one potential scenario, but the reliance on the different forms of exclusivity can depend on a number of factors.
Firstly, not all modes of exclusivity may be possible. For instance, even though one may obtain a patent and a MA, it may not be possible to obtain SPC protection. If, for example, the compound has already been the subject of a SPC, but the patent and MA have been granted for a specific use of that compound, it may not be possible to obtain a SPC. In such a situation, in the context of the timeline above, the protection afforded by RDP and/or orphan drug market exclusivity may be particularly important because it expires later than the patent.
Secondly, the timing of the MA may have a significant impact. This may be illustrated in the following timelines.
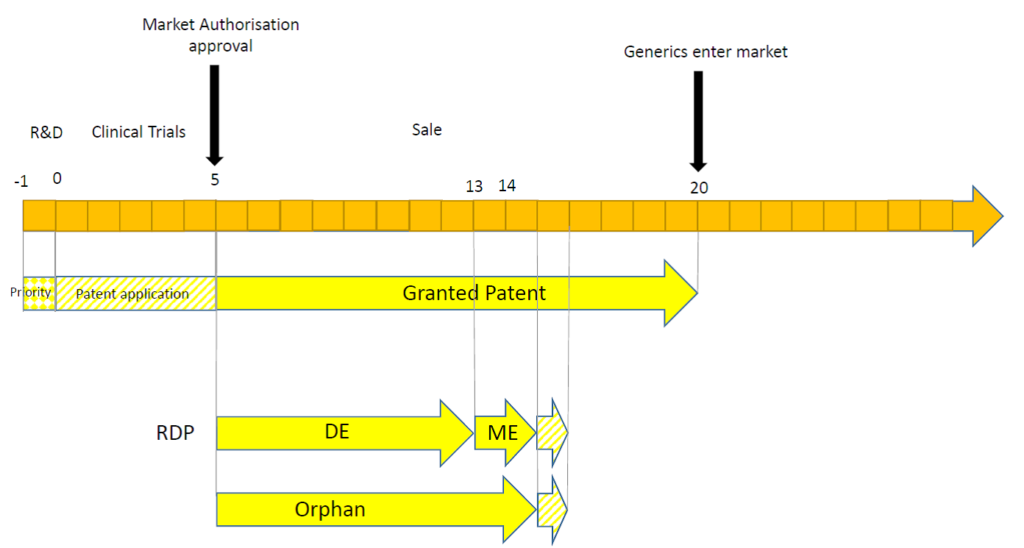
In this scenario, the MA is granted comparatively quickly. SPC protection is not relevant because the term of an SPC is equal to the period of time between the filing date of the patent and the date of the MA (i.e. 5 years), minus five years (i.e. 0 years). Furthermore, the patent term is considerably longer than that afforded by RDP and by orphan drug market exclusivity.
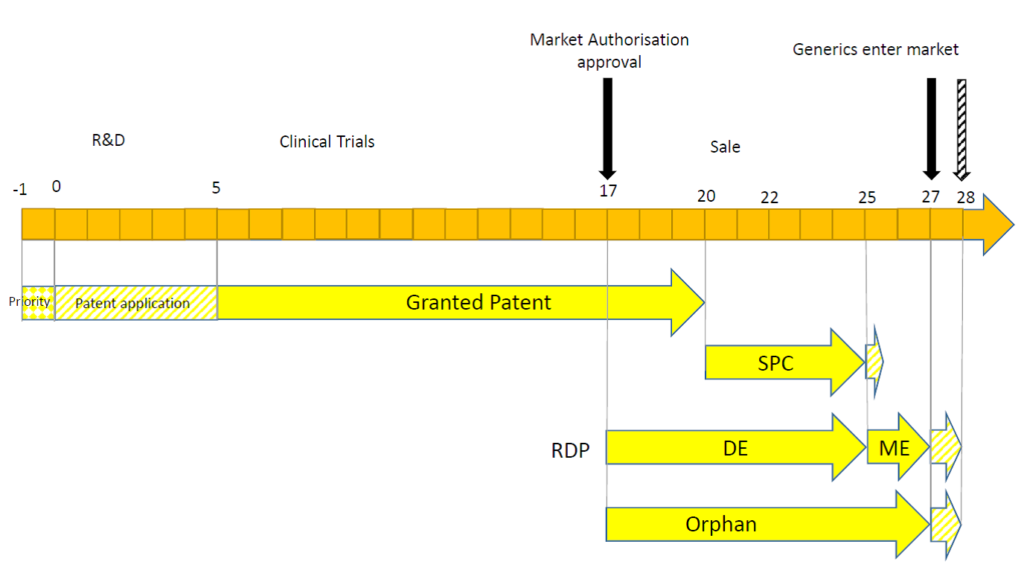
In this example, the MA is granted deep into the patent term. The full 5 years of SPC protection is possible, but the exclusivity afforded by the RDP or orphan drug market exclusivity exceeds the maximum term of the SPC.
The hypothetical scenarios above provide a flavour of how different types of exclusivity may operate to keep competitors, such as generic companies, off the market, and how the significance of the different types of exclusivity can vary depending on a number of factors, such as when a MA is obtained.
This article is for general information only. Its content is not a statement of the law on any subject and does not constitute advice. Please contact Reddie & Grose LLP for advice before taking any action in reliance on it.


